Zacks Small Cap Analysis – BCLI: Citizen’s Petition Filed Requesting the FDA Approve NurOwn™… – Cyber Tech
By David Bautz, PhD
READ THE FULL BCLI RESEARCH REPORT
Enterprise Replace
Citizen’s Petition Filed Requesting FDA Approve NurOwn™
On July 3, 2025, a Citizen’s Petition was filed with the U.S. Meals and Drug Administration during which the petitioners, a gaggle consisting principally of amyotrophic lateral sclerosis (ALS) sufferers who’ve beforehand obtained NurOwn therapy, are requesting 1) that the company invite BrainStorm Cell Therapeutics, Inc. (NASDAQ:BCLI) to re-submit its Biologics License Software (BLA) for NurOwn, and a pair of) request that the FDA exert its “regulatory flexibility” and grant Accelerated Approval or new Conditional Approval of NurOwn for the therapy of ALS with a Section 4 confirmatory research with requirement of sufferers’ participation in a biorepository with a pure historical past and exposome database. The total 300+ web page petition has been assigned Docket ID FDA-2025-P-2109 and may be accessed right here.
We spoke with BrainStorm’s administration they usually confirmed that the corporate was not concerned in crafting the petition they usually solely not too long ago discovered about it. Based mostly on how massive and intensive the doc is we estimate it has seemingly been in manufacturing for not less than a yr.
The petition contains the entire information that has been printed on NurOwn together with new information compiled from sufferers that participated within the Expanded Entry Program (EAP), together with:
- 100% 5-year survival in comparison with an anticipated 20% five-year survival fee based mostly on ALS pure historical past information
- Total survival (OS) improved by 5.5 months in comparison with matched controls as of 2022
- Tracheostomy-free survival that was as a lot as 5 years longer than median ALS pure historical past
- Prolonged intervals of Development-free survival (PFS)
- Lengthy-term slowing of ALS development by as a lot as 85%
- Important influence on respiratory perform as measured by delayed time-to-event for non-invasive air flow (NIV)
Whereas a full abstract of the petitioner’s arguments is past the scope of this report, we are going to spotlight what we imagine to be essentially the most salient information factors under.
Expanded Entry Program Knowledge
For the reason that Advisory Committee assembly for NurOwn in September 2023, the petitioners have compiled information for the ten individuals within the EAP utilizing a number of information sources. The next desk summarizes the Total Survival (OS) information for these ten individuals.
Included within the desk is the anticipated median survival information for an ALS affected person from the primary symptom onset (30 months) and analysis (18 months) (Traxinger et al., 2013; Knibb et al., 2016). The median total survival for the ten individuals within the EAP was 85.0 months from the primary symptom onset and 78.0 months from analysis, each of that are immense enhancements over what can be anticipated in a gaggle of ALS sufferers. As well as, all 10 sufferers lived for longer than 5 years.
To corroborate the survival information, we carried out an examination of assorted publicly obtainable data sources, which exhibits that life expectancy for these recognized with ALS is often 2-5 years from when signs first seem, nonetheless there’s a extensive diploma of variability:
- Typical survival instances from onset of signs seems to be influenced by ethnicity and may vary from 24 months for these from Northern Europe to 48 months to these from Central Asia (Marin et al., 2016).
- The Facilities for Illness Management estimates that “most individuals with ALS dwell on common between 2 to five years after signs develop” (CDC Nationwide ALS Registry).
- The Nationwide Institute of Neurological Problems and Stroke (NINDS) states that “most individuals with ALS die…often inside three to 5 years of signs first showing. However about 1 in 10 individuals survive for 10 years or extra.” (NINDS ALS).
Particularly analyzing the speed of sufferers that survive higher than 5 years exhibits that:
- A 2012 overview cited a number of ALS research with a median survival of 20-36 months for population-based research, five-year survival charges starting from 10-20%, and 5%-10% of ALS sufferers surviving for greater than 10 years (Chio et al., 2009).
- A research out of Olmsted County, Minnesota discovered that of the 94 sufferers that have been recognized with ALS between 1925 and 2004, 14% survived for 5 years or extra following symptomatic onset whereas the imply survival for all 94 sufferers was 2.95 years (Mateen et al., 2010).
Based mostly upon the above information, it seems most unlikely that 10/10 ALS sufferers within the EAP surviving for higher than 5 years occurred by probability. These real-world information symbolize a clinically significant commentary and make a compelling argument that NurOwn is having a constructive influence on the EAP affected person’s illness trajectory.
Along with the spectacular OS information, the information for “tracheostomy-free” survival (e.g., the time till a affected person is placed on a respiration machine by means of a tracheostomy) is maybe much more spectacular. The OS information proven above contains the truth that all 10 individuals within the EAP have been dwelling “trach-free” a minimal of 5 years from symptom onset. 4 of the EAP individuals have handed away after hitting the five-year survival threshold, nonetheless even these people lived a median of 5.5 years trach-free from symptom onset, which is almost three years longer than the anticipated median trach-free survival.
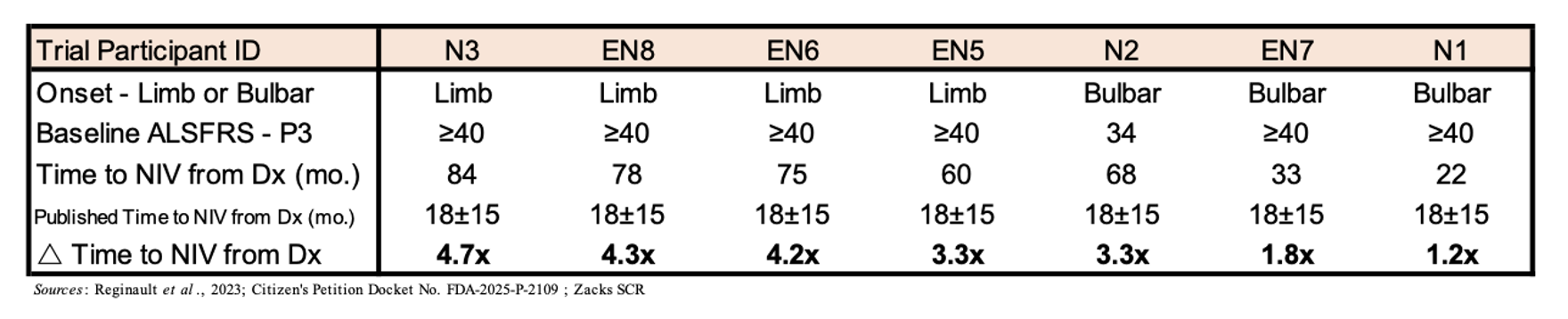
The main explanation for loss of life in ALS sufferers is respiratory failure, which is brought on by the lack of motor neurons within the phrenic nerve, which innervates the diaphragm and is crucial for respiratory perform. Thus, the flexibility to keep away from assisted respiration, whether or not within the type of non-invasive air flow (NIV) or tracheostomy, improves each the affected person’s high quality of life and probably their OS. Within the desk under are information compiled for seven EAP individuals exhibiting the time to NIV from analysis. Whereas there’s restricted information from massive research concerning the median time to NIV, a 2023 research from France confirmed that the imply time from analysis to NIV initiation in a cohort of 265 ALS sufferers (half with bulbar onset illness) was 15±18 months (Reginault et al., 2023). Thus, the truth that initiation of NIV for the EAP sufferers is orders of magnitude higher than that reported within the literature is proof that NurOwn therapy could also be having a constructive influence on sufferers’ respiratory perform.
A number of Pathways to Approval
The petitioners imagine there are a number of pathways by which NurOwn may very well be authorised if the company determined to take a brand new have a look at the “totality of the proof”, significantly the brand new information from the EAP that was not evaluated as a part of the Advisory Committee assembly in September 2023.
- First, the brand new survival information seemingly meets the company’s “substantial proof” threshold. Whereas there have been solely 10 individuals within the EAP, the standard of the information is such that the petitioners imagine it overrides the truth that the inhabitants was small.
- Second, if it doesn’t meet the “substantial proof” threshold, the petitioners imagine the Section 3 information together with the EAP information seemingly meet the “cheap chance” threshold for accelerated approval. The unprecedented “time to tracheostomy” and “time to NIV” are very more likely to moderately predict a clinically significant end result on mortality, as a consequence of the truth that respiratory perform is the largest survival predictor in ALS.
- Third, the biomarker information beforehand disclosed by the corporate (see right here) seemingly helps using the accelerated approval pathway. The biomarker information reveal goal engagement throughout varied illness pathways, together with neuroinflammation, neurodegeneration, and neuroprotection.
- Lastly, new FDA Commissioner Dr. Martin Makary has mentioned his intention to provoke a “new pathway” for approving uncommon illness medicine, with approval based mostly on a “believable mechanism”. This pathway can be reserved for uncommon or incurable illnesses that have an effect on a small variety of people (e.g., ALS) and medicines authorised below this pathway would come with a affected person surveillance system. Whereas the specifics of that pathway are nonetheless being codified, the petitioners imagine NurOwn would match with the imaginative and prescient for that program and BrainStorm already has all the pieces in place to start a Section 4 post-marketing research by means of its preparation for a Section 3b trial.
Conclusion
The Citizen’s Petition requesting that the FDA use its “regulatory flexibility” to approve NurOwn is an attention-grabbing growth for BrainStorm and gives the corporate with an immense alternative. Having reviewed the complete doc, we imagine the petitioners make a compelling argument for why NurOwn must be conditionally authorised whereas a Section 4 research is performed. At this level, BrainStorm continues to be planning to conduct a Section 3b trial, as per the SPA with the FDA, and in line with its newest convention name all that’s left to do is acquire the mandatory funding earlier than that trial may be initiated. Thus, if conditional approval have been granted the corporate can be able to provoke a confirmatory Section 4 trial virtually instantly.
How the FDA will reply to this petition is unknowable at this level, nonetheless based mostly on our studying of the petition we imagine there’s a excessive chance that the corporate might be invited to resubmit the BLA for NurOwn, together with the beforehand undisclosed EAP information. Based mostly on this, now we have adjusted our mannequin by rising the likelihood of approval for NurOwn, which has elevated our valuation to $17 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to obtain our articles and experiences emailed on to you every morning. Please go to our web site for added data on Zacks SCR.
DISCLOSURE: Zacks SCR has obtained compensation from the issuer straight, from an funding supervisor, or from an investor relations consulting agency, engaged by the issuer, for offering analysis protection for a interval of at least one yr. Analysis articles, as seen right here, are a part of the service Zacks SCR gives and Zacks SCR receives quarterly funds totaling a most price of as much as $40,000 yearly for these companies supplied to or concerning the issuer. Full Disclaimer HERE.